Have you ever wondered how modern chemistry has evolved to such precise and reliable measurements? What tools do scientists use to ensure their findings are accurate and reproducible? In the realm of analytical chemistry, one such groundbreaking tool has been the Kjeldahl flask. But what exactly is a Kjeldahl flask, and why has it become a cornerstone in chemical experimentation?
Kjeldahl flasks are specialized pieces of laboratory glassware used primarily for nitrogen determination in substance analysis. These flasks have revolutionized how chemists measure nitrogen content, a crucial parameter in fields like biochemistry, environmental science, and food science. Their unique design and functionality have ushered in a new era in chemical experimentation.
Stay tuned as we delve deeper into the world of Kjeldahl flasks and their impact on scientific research.

What is the Kjeldahl Flasks?
The “Kjeldahl Flasks” are named after the Danish chemist Johan Kjeldahl, who developed the Kjeldahl method in 1883. This method, crucial for determining nitrogen content in organic compounds, involves a specific process requiring a unique flask design. Kjeldahl’s method was a significant advancement in analytical chemistry, particularly in protein analysis in various fields like agriculture and food science. The flasks used in this method, characterized by their distinct shape ideal for boiling and minimizing liquid loss, were hence named in honor of their inventor, Johan Kjeldahl.
Design and Construction of Kjeldahl Flasks
Kjeldahl flasks are specifically designed pieces of laboratory glassware used primarily for nitrogen determination in substances through the Kjeldahl method. Their construction features several key elements:
- Material: They are usually made from borosilicate glass, which is highly resistant to thermal shock and chemical corrosion. This makes them suitable for the harsh conditions of the Kjeldahl digestion process.
- Shape: Kjeldahl flasks typically have a round bottom with a long, narrow neck. The round bottom allows for even heating and boiling, while the narrow neck helps to prevent the loss of liquid through evaporation and aids in the containment of gases produced during digestion.
- Size and Volume: These flasks come in various sizes, often ranging from 250 mL to 800 mL, to accommodate different sample sizes and the volume of acid required for digestion.
- Durability: The design and material of Kjeldahl flasks make them durable and suitable for repeated use under high temperatures and with corrosive chemicals like concentrated sulfuric acid.
This construction allows Kjeldahl flasks to effectively facilitate the chemical digestion of samples, a crucial step in the Kjeldahl method for determining nitrogen content.
Why are Kjeldahl Flasks Essential in the Kjeldahl Method?
Kjeldahl flasks are essential in this method due to their unique shape and durability. They are designed to withstand the high temperatures and corrosive chemicals used during the digestion process. The narrow neck of the flask minimizes the loss of ammonia, ensuring accurate measurements.
Characteristics of the Kjeldahl Flask
A Kjeldahl flask is a specially designed and purposed glassware used primarily in chemistry experiments and analyses. Its characteristics include:
- Neck Design: The Kjeldahl flask typically features a ground glass neck for the addition of liquid reagents or buffers. This design allows for controlled flow during reagent addition and facilitates the easy introduction of various chemicals.
- Spherical Sidearm Connection: It has a spherical sidearm connected to a rubber stopper, forming a gas generation apparatus. This feature enables the effective collection of gases like carbon dioxide and oxygen during experiments and helps maintain pressure balance, avoiding experimental results’ alteration due to pressure changes.
- Colorless, Dry Material: Kjeldahl flasks are usually made of colorless, dry material, ensuring the accuracy of experimental results. The dry nature also makes them suitable for long-term sample storage, particularly when containing samples.
- Good Sealing Property: These flasks have excellent sealing properties, effectively preventing gas or liquid leakage. This not only enhances the accuracy of experiments but also ensures no environmental contamination during the process.
- High-Temperature Resistance: Made from high-temperature resistant glass, Kjeldahl flasks can withstand higher temperatures, which is beneficial for experiments requiring high-temperature heating for certain chemical reactions.
- Wide Applicability: Kjeldahl flasks are suitable for various types of chemical experiments, including organic, inorganic, and analytical chemistry, making them widely applicable in different fields.
- High Safety: The design and material of Kjeldahl flasks ensure high safety during use. Their robustness and durability provide confidence to experimenters, reducing the risk of injury due to accidental drops or other mishaps.
- Easy Observation: Being typically transparent, they allow experimenters to easily observe the reactions taking place, aiding in a better understanding of the experimental process and timely adjustment of conditions.
- Ease of Cleaning: Their design facilitates easy cleaning. Residues inside the flask can be easily removed with appropriate detergents and methods, ensuring accuracy for subsequent experiments.
- Durability: Made of high-quality glass, Kjeldahl flasks are long-lasting. With proper use and maintenance, they can remain in good condition for a long time, providing a stable and reliable environment for experimenters.
In summary, Kjeldahl flasks are vital laboratory glassware in chemistry labs, featuring unique designs and multiple characteristics. These attributes make them widely used in chemistry experiments and analyses, offering accurate, safe, and reliable conditions for experimenters. With correct use and maintenance, Kjeldahl flasks become an indispensable part of a chemistry laboratory.
What is the function of Kjeldahl apparatus
Kjeldahl flasks play a pivotal role in the field of analytical chemistry, primarily used for the determination of nitrogen content in various substances. Their utility spans across multiple industries and scientific disciplines. Here’s an overview of their functions and applications:
- Function in Nitrogen Determination: The primary role of Kjeldahl flasks is in the Kjeldahl method, a process for quantifying the nitrogen content in organic and inorganic samples. This method involves digesting the sample in the flask with sulfuric acid, converting nitrogen into a measurable form.
- Agriculture: In agriculture, they are used to analyze soil and fertilizer compositions, helping in understanding the nutrient content and guiding effective fertilizer application.
- Food and Beverage Industry: Kjeldahl flasks are crucial in determining the protein content in food and beverages, which is essential for nutritional labeling and quality control.
- Environmental Science: They assist in assessing water quality by measuring the nitrogen levels in water bodies, which is vital for monitoring pollution and environmental health.
- Pharmaceuticals: In pharmaceuticals, these flasks are used for quality control testing, ensuring that products comply with the required standards and regulations.
- Research and Academia: They are widely used in academic research for various chemical analyses, teaching students about fundamental analytical techniques.
The versatility and reliability of Kjeldahl flasks in these applications underscore their significance in scientific and industrial realms.
The Role and Proper Usage of Kjeldahl Flasks
Kjeldahl flasks are pivotal in enhancing the precision and efficiency of chemical analyses. This guide first explores their impact on the field and then details the essential steps for their correct utilization in laboratory settings.
How Have Kjeldahl Flasks Advanced Chemical Experimentation
- Accuracy: Kjeldahl flasks allow for precise measurements of nitrogen, which is vital in many scientific disciplines.
- Reproducibility: The standardized design of these flasks ensures consistent results across different experiments and laboratories.
- Versatility: They are used in various fields, from environmental testing to quality control in food production.
Effective Usage and Maintenance of Kjeldahl Flasks
Using a Kjeldahl flask properly is essential for accuracy and safety in chemical experiments and analyses. Here’s a guide on how to use it correctly:
Preparation Before Use
- Cleaning: Ensure the Kjeldahl flask is clean and dust-free before use. Clean the body, neck, and mouth with detergent and rinse thoroughly with water.
- Drying: Dry the flask in an oven or wipe it with a clean cloth. Make sure there’s no residual moisture inside to avoid affecting the experimental results.
- Weighing: If necessary, weigh the flask using a balance to record data during the experiment.
During Use
- Adding Reagents: Add the required reagents to the flask as per the experimental protocol. Exercise caution to prevent spills or skin contact.
- Shaking the Flask: Gently shake the flask to ensure thorough mixing of the reagents. Avoid shaking too vigorously to prevent breakage.
- Temperature Control: Control the temperature of the Kjeldahl flask according to the experiment’s needs, using heating or cooling equipment as necessary.
- Observing the Reaction: Closely monitor the reaction inside the flask. If any abnormality occurs, stop the experiment immediately and take appropriate measures.
- Data Recording: Record relevant experimental data during or after the experiment for subsequent analysis and calculation.
Post-Use Cleaning and Storage
- Cleaning: Immediately clean the flask with detergent after the experiment. Pay special attention to the neck and mouth to avoid residues affecting future experiments.
- Drying: Dry the cleaned flask for future use.
- Storage: Store the flask in a dry, cool place away from direct sunlight or high temperatures to prevent breakage or deformation. Ensure the lid is tightly closed to keep out dust and impurities.
Safety Precautions
- Inspect for Damage: Ensure the flask is intact before use. Replace it if damaged.
- Avoid Burns and Frostbite: Take safety precautions to prevent burns or frostbite during handling.
- Emergency Protocol: In case of any accident, stop the experiment immediately and seek professional help.
- Chemical Exposure: Do not store the flask near strong acids, bases, or other corrosive substances to avoid dangerous chemical reactions.
- Fire Safety: Be cautious when using heating equipment. Keep it away from flammable and combustible materials.
- Ventilation: Conduct experiments in a well-ventilated area to avoid prolonged exposure to harmful gases or substances.
- First Aid: In case of skin or eye contact with reagents, rinse immediately with plenty of water and seek medical help.
- Cleanup: Clean the area promptly after the experiment, ensuring no hazardous materials or waste are left behind.
Proper use of a Kjeldahl flask is a critical component of chemical experiments and analyses. It’s important to operate safely, accurately, and methodically to ensure the reliability and accuracy of experimental results. Patience and attention to detail are key to preventing accidents due to improper handling.
Maintenance and Calibration: Ensuring Accuracy and Longevity of Kjeldahl Flasks
Proper maintenance and calibration are essential for ensuring the accuracy and longevity of Kjeldahl flasks in chemical experimentation. Here’s a guide on how to effectively care for and maintain these vital pieces of laboratory equipment:
- Regular Cleaning: After each use, Kjeldahl flasks should be thoroughly cleaned to remove any residues. Use appropriate laboratory detergents and rinse with distilled water. For stubborn residues, a soak in a mild acid solution might be necessary.
- Handling with Care: Despite their durability, Kjeldahl flasks are made of glass and can break if mishandled. Always use appropriate safety equipment, like gloves and eye protection, and handle the flasks with care to prevent accidents.
- Storage Conditions: Store the flasks in a dry, safe place. Ensure they are properly supported and not subject to extreme temperatures or direct sunlight, which can cause stress to the glass.
- Regular Inspection: Periodically inspect the flasks for any signs of damage, such as cracks or chips, especially around the neck and base. Damaged flasks can compromise the safety and integrity of experiments and should be replaced.
- Calibration for Volumetric Accuracy: If Kjeldahl flasks are used for volumetric measurements, they should be calibrated regularly. This can be done using a standard solution and comparing the measured volume to the flask’s markings. Any discrepancies should be noted and considered in experimental calculations.
- Chemical Compatibility: Be mindful of the chemicals used in the Kjeldahl flasks. Some chemicals may react with glass or cause etching over time. Understanding the chemical compatibility is essential for maintaining the integrity of the flask.
- Avoiding Thermal Shock: When heating Kjeldahl flasks, increase the temperature gradually to avoid thermal shock, which can weaken or crack the glass. Similarly, allow the flasks to cool down naturally after heating.
- Proper Use of Equipment: Always use Kjeldahl flasks as intended. Avoid using them for applications they are not designed for, as this can lead to inaccurate results or damage to the flask.
- Record Keeping: Maintain a logbook for each flask, noting down its usage, cleaning, and any maintenance performed. This helps in tracking the flask’s condition and identifying when it’s due for replacement.
By following these maintenance and calibration guidelines, the reliability and accuracy of experiments using Kjeldahl flasks can be significantly enhanced, ensuring they remain a valuable asset in the laboratory for years to come.
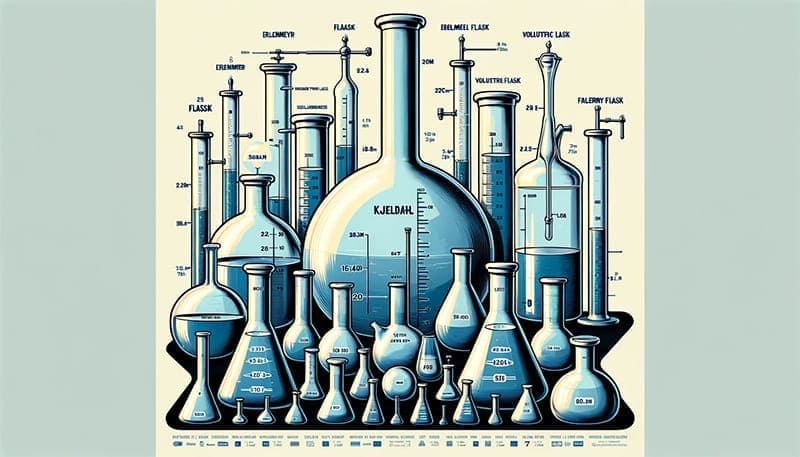
Comparing Kjeldahl Flasks with Other Laboratory Flasks
When working in a laboratory, it’s crucial to understand the differences between various types of flasks, as each is designed for specific uses. Below, we compare Kjeldahl flasks with other common types of flasks, such as Erlenmeyer flasks and volumetric flasks, to highlight their unique features and applications.
| Flask Type | Description | Applications | Heat Resistance | Durability & Chemical Resistance | Accuracy & Precision | Closure Types | Ease of Cleaning | Standard Sizes |
|---|---|---|---|---|---|---|---|---|
| Kjeldahl Flask | Round bottom, long narrow neck. Resistant to thermal shock and corrosion. | Nitrogen determination in substances. | High | High durability, good chemical resistance. | Moderate | Usually open, can fit certain stoppers. | More challenging due to narrow neck. | 250 mL to 800 mL |
| Erlenmeyer Flask | Conical shape, flat bottom, wide mouth. | Mixing, heating, reactions in the lab. | High | Varies based on material, generally good. | Low (not for precise measurements) | Open, can fit various stoppers or caps. | Easier due to wide mouth. | 50 mL to several liters |
| Volumetric Flask | Flat bottom, long neck, calibration mark. Comes with a stopper. | Precise solutions preparation, titration. | Moderate to High | Good, but more fragile due to precision. | High (calibrated for precision) | Typically comes with a stopper. | Easy, but requires care for the narrow neck. | 1 mL to 2 liters |
Understanding these differences is key to selecting the right flask for your specific laboratory needs, whether it’s for precise chemical analysis or general mixing and reactions.
Each type of flask – Kjeldahl, Erlenmeyer, and Volumetric – has distinct characteristics and applications. The choice of flask depends on the specific requirements of the experiment or analysis being conducted in the laboratory.
Environmental and Safety Considerations: The Use of Kjeldahl Flasks
While Kjeldahl flasks are essential in analytical chemistry, their use raises important environmental and safety considerations, particularly concerning the handling and disposal of hazardous chemicals. Here’s a detailed look at these aspects:
- Handling Hazardous Chemicals: The Kjeldahl method typically involves corrosive substances like concentrated sulfuric acid. Proper handling protocols must be followed, including the use of personal protective equipment (PPE) such as gloves, lab coats, and eye protection.
- Fume Hood Usage: Many of the reactions in the Kjeldahl method release toxic fumes. Performing these reactions in a fume hood is crucial to prevent inhalation of harmful gases.
- Chemical Spill Management: Laboratories should have clear procedures for dealing with spills of hazardous chemicals. Quick and safe response is essential to minimize risks to personnel and the environment.
- Waste Disposal: The disposal of waste from the Kjeldahl process, which may contain residual acids and nitrogen compounds, needs to adhere to environmental regulations. This often involves neutralizing acids before disposal and ensuring that nitrogen compounds are disposed of in a way that doesn’t harm aquatic ecosystems.
- Training and Education: Laboratory personnel must be adequately trained in the use of Kjeldahl flasks and the handling of dangerous chemicals. Regular training sessions can help in maintaining a high level of safety awareness.
- Environmental Impact Assessment: Laboratories should assess the environmental impact of their waste and find ways to minimize it. This might include recycling certain chemicals or modifying the process to reduce waste.
- Emergency Preparedness: Having a well-prepared emergency response plan for accidents, such as chemical burns or inhalation, is essential. This should include first aid measures and emergency contact information.
- Regular Safety Audits: Conducting regular safety audits helps in identifying potential hazards and implementing corrective actions to improve safety.
- Ventilation Systems: Proper ventilation in laboratories using Kjeldahl flasks is critical. Adequate ventilation systems help disperse harmful gases, reducing the risk of inhalation.
- Sustainable Practices: Exploring more sustainable and eco-friendly alternatives for the Kjeldahl method, such as using less toxic reagents or implementing recycling methods, can significantly reduce environmental impact.
By addressing these environmental and safety considerations, the use of Kjeldahl flasks and the associated chemical processes can be managed in a way that is safer for both laboratory personnel and the environment. It is crucial to balance the scientific needs with the responsibility towards health and environmental sustainability.

Regulatory and Standardization Aspects: Governing Kjeldahl Flasks and Method
The use of Kjeldahl flasks and the Kjeldahl method in various industries is subject to certain international standards and regulations to ensure accuracy, safety, and consistency. Here’s an overview of these regulatory and standardization aspects:
- ISO Standards: The International Organization for Standardization (ISO) has set specific standards for methods of analysis, including those that use Kjeldahl flasks. For instance, ISO 5983-2:2009 specifies a method for the determination of nitrogen content in animal feeding stuffs using the Kjeldahl method.
- ASTM International: ASTM International, formerly known as American Society for Testing and Materials, provides standard protocols for a wide range of materials and processes, including the Kjeldahl method. These standards ensure that the method is carried out uniformly and reliably across different laboratories.
- AOAC International: AOAC International, a body dedicated to developing analytical standards, has approved specific Kjeldahl-based methods for nutrient analysis in food and feeds. These methods are widely used in food quality control and labeling.
- Environmental Protection Regulations: In environmental testing, regulatory agencies like the U.S. Environmental Protection Agency (EPA) have specific guidelines on nitrogen determination methods, including the use of Kjeldahl flasks, for water and soil analysis.
- Food and Drug Administration (FDA) Regulations: In the pharmaceutical and food industries, the FDA sets standards for the composition and quality of products. The Kjeldahl method is often used to comply with these regulations, especially for protein content analysis.
- European Pharmacopoeia (EP) and United States Pharmacopoeia (USP): For pharmaceutical products, these pharmacopoeias include methods that rely on Kjeldahl flasks for determining various compounds’ nitrogen content, ensuring that pharmaceutical products meet the required purity standards.
- Good Laboratory Practices (GLP): Laboratories conducting experiments and analyses with Kjeldahl flasks must adhere to GLP, ensuring that the procedures are carried out in a controlled, consistent, and reliable manner.
- Safety Standards: Safety standards are crucial, especially when handling corrosive substances used in the Kjeldahl method. These include proper handling, storage, and disposal practices as per international safety guidelines.
- Calibration and Maintenance Standards: Regular calibration and maintenance of Kjeldahl flasks and associated equipment are necessary to meet the precision requirements set by these standards.
By adhering to these international standards and regulations, industries and laboratories ensure that the use of Kjeldahl flasks and the associated analytical methods are not only effective but also compliant with global best practices. This standardization is crucial for maintaining consistency and reliability in various scientific and industrial applications.
Educational Importance in Chemistry Curriculum: The Role of Kjeldahl Flasks
Kjeldahl flasks play a significant role in educational settings, particularly in university laboratories, where they serve as vital tools in teaching the fundamentals of analytical chemistry. Here’s an elaboration on their importance in the educational context:
- Hands-On Learning: The use of Kjeldahl flasks in laboratory exercises provides students with hands-on experience in conducting real-world chemical analyses. This practical approach is crucial in understanding the principles of analytical chemistry beyond theoretical learning.
- Teaching the Kjeldahl Method: The Kjeldahl method is a classic analytical technique, and learning it helps students understand nitrogen determination’s importance in various fields. Using Kjeldahl flasks, students can directly engage with this essential method, gaining insights into its application and significance.
- Understanding Chemical Processes: By using Kjeldahl flasks, students learn about chemical digestion, distillation, and titration processes. This experience is invaluable in understanding reaction mechanisms, material properties, and the precision required in chemical experiments.
- Developing Laboratory Skills: Working with Kjeldahl flasks helps students develop essential laboratory skills such as accurate measuring, proper handling of chemicals, and understanding of laboratory safety protocols.
- Exposure to Industry-Standard Equipment: Since Kjeldahl flasks are widely used in various industries, students gain experience with equipment that they are likely to encounter in their professional careers. This exposure is critical for preparing them for roles in scientific research, quality control, environmental monitoring, and more.
- Fostering Scientific Inquiry: Experiments using Kjeldahl flasks encourage students to ask questions, make observations, and engage in critical thinking. This inquiry-based learning is fundamental to the development of scientific reasoning and problem-solving skills.
- Integrating Theory and Practice: The use of Kjeldahl flasks in educational laboratories bridges the gap between theoretical knowledge acquired in lectures and practical application in the laboratory. This integration is key to a comprehensive understanding of chemistry.
- Safety Training: Working with Kjeldahl flasks involves handling hazardous chemicals and heating processes. This offers an excellent opportunity for students to learn about laboratory safety, risk assessment, and the proper response to potential hazards.
Kjeldahl flasks are not just laboratory apparatus; they are educational tools that enrich the chemistry curriculum by providing practical experience, enhancing skill development, and preparing students for future scientific endeavors. Their role in educational settings is pivotal in cultivating the next generation of chemists and researchers.
Future Trends and Innovations: Advancing Nitrogen Determination
The future of nitrogen determination, particularly in relation to the Kjeldahl method and Kjeldahl flasks, is poised for significant advancements. Here’s a look at potential technological trends and innovations that could redefine efficiency and safety in this field:
- Automation in the Kjeldahl Method: There’s a growing trend towards automating the Kjeldahl process. Future developments could include fully automated systems that handle all stages of nitrogen determination – from digestion to distillation and titration. This automation would not only increase efficiency but also reduce the risk of human error and exposure to hazardous chemicals.
- Advanced Flask Design: Innovations in Kjeldahl flask design are expected to focus on enhancing safety and durability. This might involve the use of more resilient materials or integrating safety features to prevent breakage and contain chemical spills.
- Integration with Digital Technology: Incorporating digital technology into Kjeldahl equipment could lead to more precise control of experimental conditions and real-time monitoring of reactions. This integration would also facilitate the collection and analysis of data, improving the overall reliability of results.
- Green Chemistry Approaches: As environmental concerns continue to grow, the development of greener alternatives to the chemicals currently used in the Kjeldahl method will be a key area of focus. This could involve exploring less hazardous reagents or developing methods that generate less waste.
- Enhanced Safety Protocols: Future innovations may also include advanced safety protocols, such as automated safety checks and emergency shutdown mechanisms, to protect laboratory personnel.
- Miniaturization and Portability: The development of smaller, portable Kjeldahl systems could make nitrogen determination accessible in field settings. This would be particularly beneficial in environmental monitoring and agricultural applications.
- Machine Learning and AI Integration: The application of artificial intelligence and machine learning could revolutionize nitrogen analysis by predicting outcomes, optimizing experimental conditions, and even diagnosing system malfunctions.
- Collaborative Research Platforms: Advances in technology could facilitate greater collaboration in research, with cloud-based platforms enabling scientists to share data and methodologies, thus speeding up the pace of innovation in nitrogen determination.
These future trends and innovations in the Kjeldahl method and flask design are not just about technological advancements; they represent a shift towards more efficient, safe, and environmentally conscious nitrogen determination. As these technologies develop, they will undoubtedly play a crucial role in shaping the future of analytical chemistry.
Conclusion
Dive into the world of analytical chemistry with the groundbreaking Kjeldahl method. Embrace the future of scientific innovation, and join us in exploring new frontiers in research and education. The journey of discovery is ongoing, and your engagement and curiosity are the catalysts for future advancements. Let’s continue to unravel the mysteries of chemistry together, with Kjeldahl flasks as our trusted companions in this exciting scientific endeavor.










